

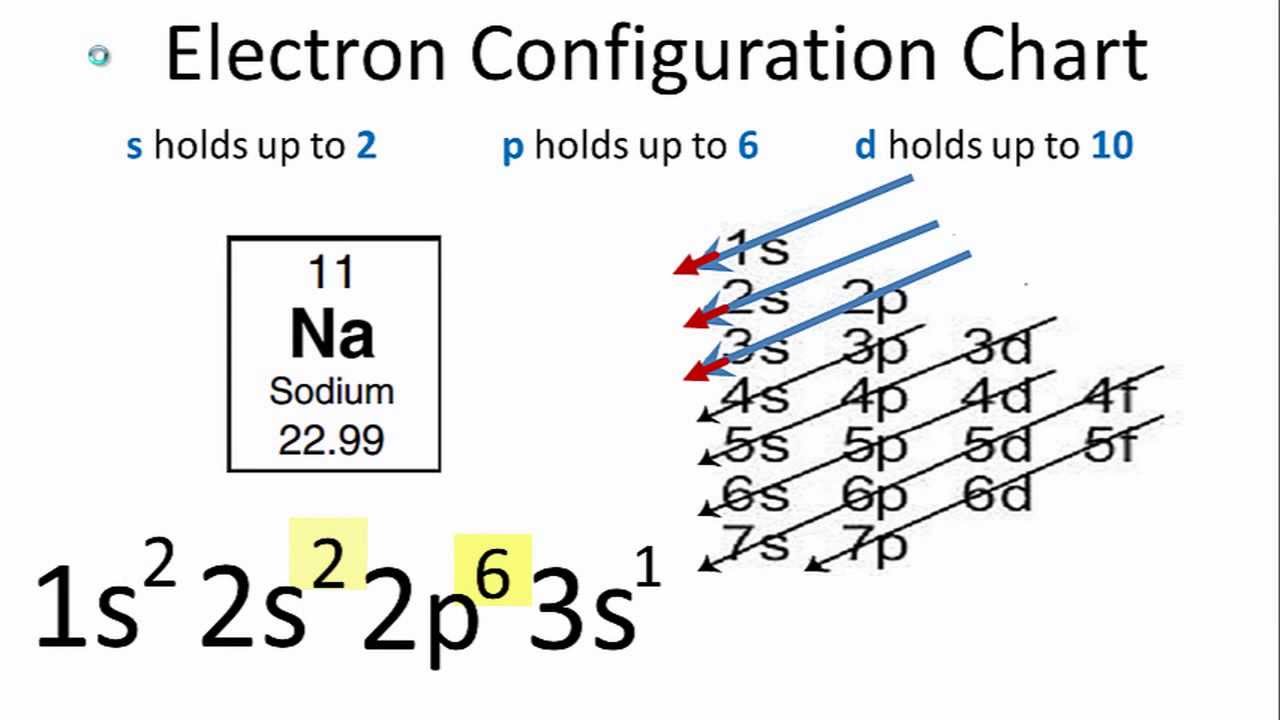
Since the first orbital shell has only two electrons, we know that Boron has two shells: one with two 1s electrons and one with three electrons from the 2s and 2p orbitals. Since its atomic number is five, we know it has five electrons and its electron configuration looks like this: 1s 22s 22p 1. For example, let's say we're looking at the element Boron (B).Besides the very first shell, which can hold only two electrons, each shell can have eight electrons (except, again, when dealing with transition metals.) This is called the Octet Rule. When we're dealing with atoms outside of the transition metals, we say that these orbitals form "orbital shells" around the nucleus, with each successive shell being further out than the ones before. As electrons are added to an atom, they fall into various orbitals according to the order given above - the first two go into the 1s orbital, the two after that go into the 2s orbital, the six after that go into the 2p orbital, and so on. For more on electron configurations, see also this article.Īssign electrons to orbital shells with the Octet Rule.You only need to change the number in the final orbital - the rest is the same since the orbitals before the final one are completely full.
#Sodium element valence electrons plus#
#Sodium element valence electrons how to#
Learn how to read an electron configuration. This means that an atom can have multiple numbers of valence electrons depending on how it is manipulated. For reasons that are a little too complex to explain here, when electrons are added to the outermost d shell of a transition metal (more on this below), the first electrons that go into the shell tend to act like normal valence electrons, but after that, they don't, and electrons from other orbital layers sometimes act as valence electrons instead.Generally, the valence electrons are the electrons in the outermost shell - in other words, the last electrons added. As electrons are added to an atom, they are sorted into different "orbitals" - basically different areas around the nucleus that the electrons congregate in.See below for a quick run-through or skip this step to get right to the answers. Understanding why transition metals don't really "work" like the rest of the periodic table requires a little explanation of the way electrons behave in atoms.

Understand that transition metals don't have "traditional" valence electrons.


 0 kommentar(er)
0 kommentar(er)
